For all the confounding changes that have come out of the coronavirus pandemic, there has been at least one clear piece of advice: wash your hands. And why is something so basic so important, and what function does it have to control the spread of this virus?
‘There are two benefits of washing your face,’ said one of the Microbiologists. First of all, by brushing them with soap and placing them under the tap, you’re actively extracting a lot of dirt and viruses and other things that may be in your hair.
Structure of the virus
The virus is called coronavirus because of the corona, the crown that protects it. We are primarily composed of protein. The next step is the exterior surface of the virus, and that’s the lipid. And the important thing about lipids is that they really interact with soap and detergent. The key target of the virus is what we call the RNA-the radioactive material or the DNA. Viruses can not replicate on their own-but they are still susceptible to soaps and detergents being destroyed, and other agents like alcohol are still very powerful.
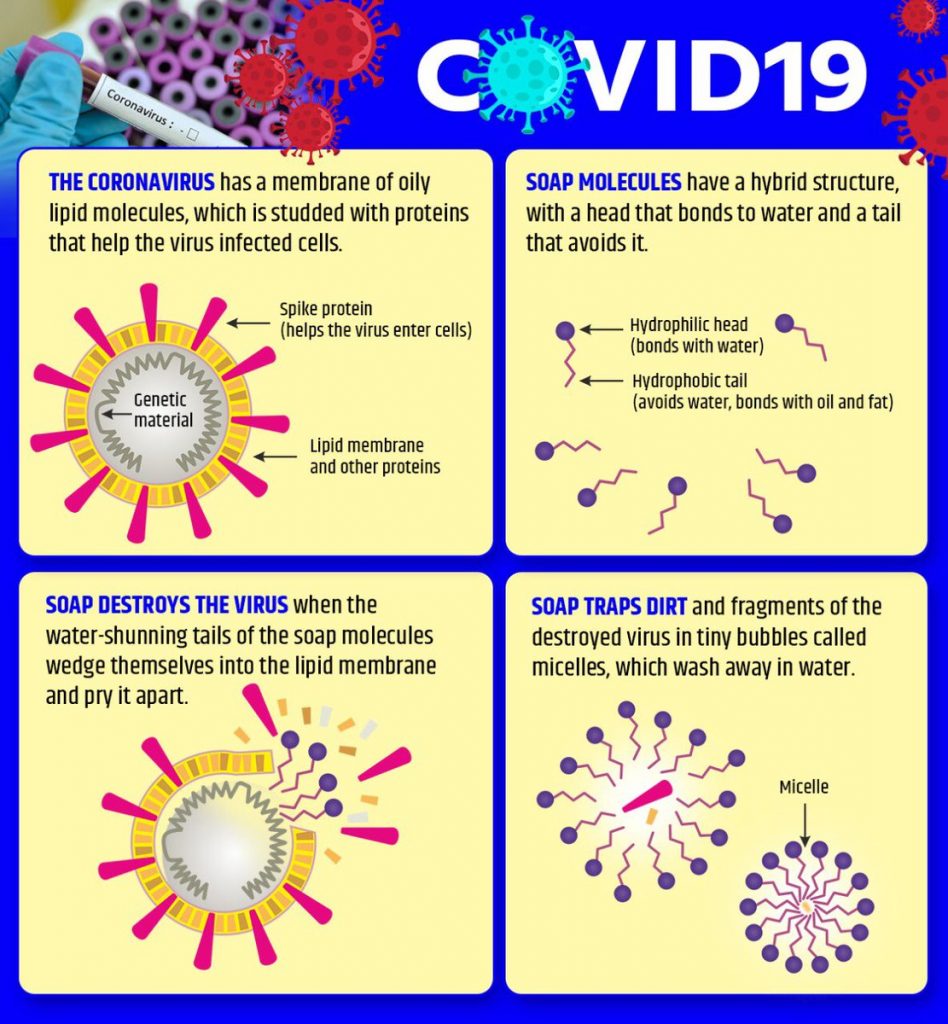
The science of soap
Viruses can be present outside the body for hours or days. Disinfectants, oils, pads, gels and alcohol-containing creams are all handy to get rid of them – but they are not as good as regular soap.
Why is soap acting so well on Sars-CoV-2, coronavirus, and indeed other viruses? The short story: since the virus is a self-assembled nanoparticle where the weakest component is the lipid (fatty) bilayer. Soap dissolves the fat membrane and the virus falls apart like a house of cards and dies – or better, we would say it is dead because the viruses aren’t even alive.
The somewhat longer version is that most viruses consist of three main building blocks: ribonucleic acid (RNA), protein and lipids. A virus-infected cell produces all of these building blocks, which then naturally combine to create a virus. Critically, there are no heavy covalent bonds binding these units together, which ensures that you do not actually require tough chemicals to isolate these units. When an infected cell dies, all these new viruses will emerge to kill other cells. Many wind up in the airways of the body, too.
If you cough, or particularly if you sneeze, tiny droplets will travel up to 10 meters from the airways. The larger ones are thought to be the main coronavirus carriers and can be at least two meters away. Such small droplets stop on the surface and sometimes dry up easily. Yet the viruses are still around. Human skin is the perfect surface for a virus. It is “normal” and proteins and fatty acids interact with the virus in the dead cells on the surface. When you reach, say, a steel surface with a virus particle on it, it sticks to your skin and is passed to your hands. If you touch your face, particularly your eyes, nostriles or mouth, you can get infected. And it turns out that most people brush their faces once every two to five minutes. Wash the virus off with water on its own could work. But water is not good at dealing with solid, sticky interactions between the skin and the virus. The water is not enough.
The soapy water is totally different. Soap contains fat-like compounds known as amphiphiles, some of which are structurally very similar to lipids in the membrane of the virus. Soap molecules “compete” with the lipids in the nucleus of the virus. That is more or less how soap often extracts natural soil from the face. Soap not only loosens the “glue” between the virus and the flesh, but also velcro-like attachments that bind together proteins, lipids and RNA in the virus.
Alcohol-based products, which include nearly all disinfectants, contain high-percent alcohol solvent (usually 60-80 per cent ethanol) and kill viruses in a similar way. Yet soap is easier because you only need a comparatively limited quantity of soapy water, which, with pressure, will quickly cover your whole side. Whereas you need to actually soak the virus in ethanol for a short period, cleaning or rubbing a solvent on your hands does not guarantee that you soak every corner of your skin in your hands efficiently enough. Yeah, soap is the safest, but please use an alcohol-based sanitizer when soap is not feasible or convenient.
The effectiveness of hand sanitizers
“Over the last 10, 20 years, we have usually used hand sanitizers in hospitals,” several doctors said. There are two explanations for this. Alcohol is also effective at killing viruses and bacteria, even viruses that don’t have lipid envelopes. Even, you don’t need a sink near you, so you can do it right at the bedside, and there are realistic hand sanitizer applications.
There’s probably not a lot of difference between soap and water and hand sanitizers when you’re at home-the sanitizer would be more useful in an area where you don’t have easy access to a sink or water.
Making your own hand sanitizers
Alcohol of any sort works-but with 100% alcohol, it doesn’t work as well. It also needs to be mixed with water or some other water-containing substance in such a manner that it is between 60 and 70 per cent-and this is the concentration of most sanitizers marketed commercially.